Our certifications
Terminter boasts awards, patents and certifications inherent in its business.
Compliance checks conducted by a third-party body enable our company to demonstrate to the Market that its production processes and materials used meet the legislative requirements correctly.
ICIM SpA is an accredited certification body whose mark represents an important "sign" for Client Organizations, their management system and their products or services. In addition to being accredited in Italy with ACCREDIA, ICIM has consolidated its presence abroad, operating directly or through bilateral or multilateral agreements by both issuing certifications and conducting part two and part three inspections. In the food & water contact field, it is involved in the accredited certification of food contact materials and objects (MOCA) and drinking water contact products and components (CAP).
The certification of products and components in contact with drinking water (CAP) is dedicated to all manufacturers of products and components that come into contact with water intended for human consumption to avoid risks from substandard releases to ensure quality and healthiness. Compliance audits conducted by ICIM enable companies to demonstrate to the market that their production processes and materials used meet legislative requirements.
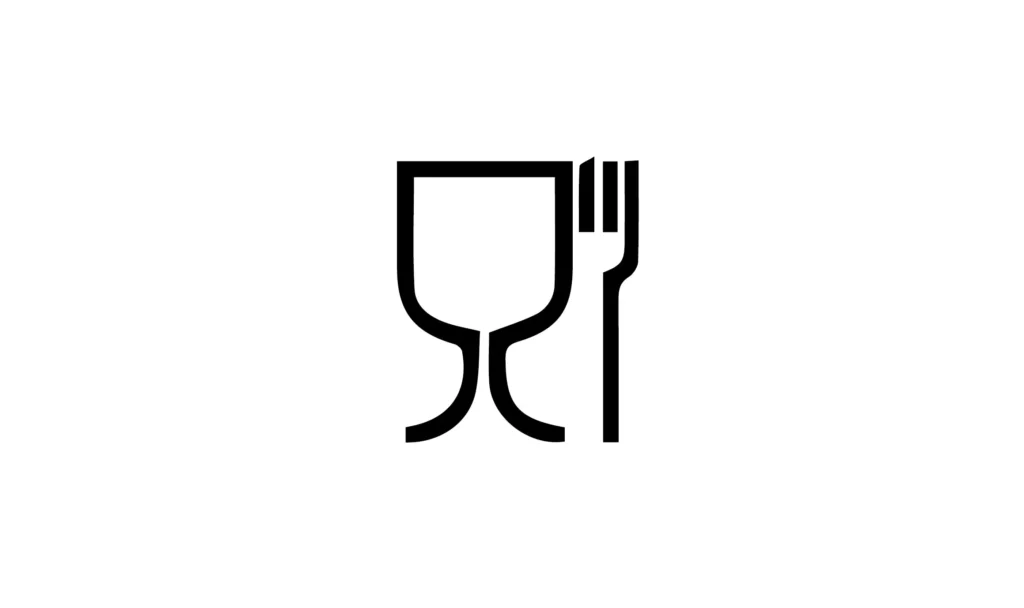
The European Regulation no. 1935/2004 defines food contact materials and articles (MOCAs) as all articles that are intended to come into contact (or are expected to come into contact) with food under normal conditions of use. It stipulates that materials and articles intended to come into contact, directly or indirectly, with food must be sufficiently inert to exclude the transfer and/or transfer to food of substances in quantities that endanger human health.
By following a methodological approach based on risk assessment, ICIM S.p.A. supports our company in equipping itself with a quality system that complies with the application of good manufacturing practices in the processes of design and production of plants and machines intended for food contact and maintenance of products in contact with food substances
BUREAU VERITAS is a leading global certification body in inspection, laboratory verification and certification services. It is accredited by more than 60 international bodies, thus being able to offer accredited verification and certification services anywhere in the world. As an accredited certification body, Bureau Veritas certifies that management systems, services and people comply with specific standards. The certifications issued by it enable our company to access new markets, strengthen its corporate reputation, and ensure the efficiency and quality of its operations.

ISO 9001 Certification is an international standard applicable by all organizations, operating in any business sector. It aims for customer satisfaction and continuous improvement of the organization's performance through a certified Quality Management System.
As a company certified according to the ISO 9001 standard, Terminter S.r.l:
- Demonstrates commitment to quality and customer satisfaction,
- Ensures that the products and services offered effectively take into account customer needs and cogent and regulatory requirements
- allows the company's progress toward continuous improvement of its market performance to be measured by using a benchmark standard against which to compare itself
- helps improve the company's organizational performance.

Terminter continues on the path of certifications by conforming to the UNI EN ISO 22000 standard for what concerns food safety management systems. This voluntary membership confirms its desire to maintain itself at the highest level in its field in order to distinguish itself by the high degree of reliability of its devices. ISO 22000 enables all companies involved in the food supply chain, either directly or indirectly, to accurately identify the risks to which they are exposed and manage them effectively.

Since 2013, it has also created a medical device section, obtaining ISO 13485 Certification; specifically, it enables our company to demonstrate its ability to provide Medical Devices and related services that comply with customer requirements and regulatory requirements applicable to such medical devices. Terminter has expanded that medical production quality standard to manufacture all devices, not just medical devices, by elevating the entire production process to more stringent and strict regulatory systems.







